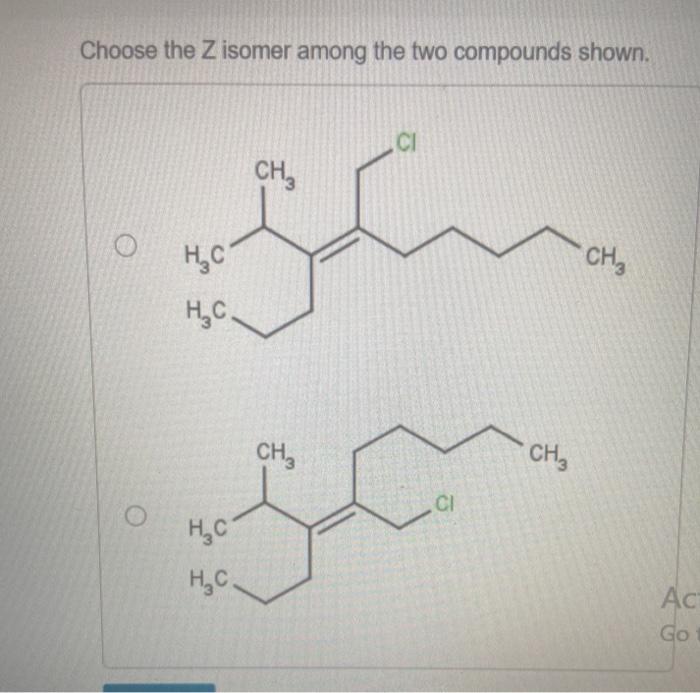
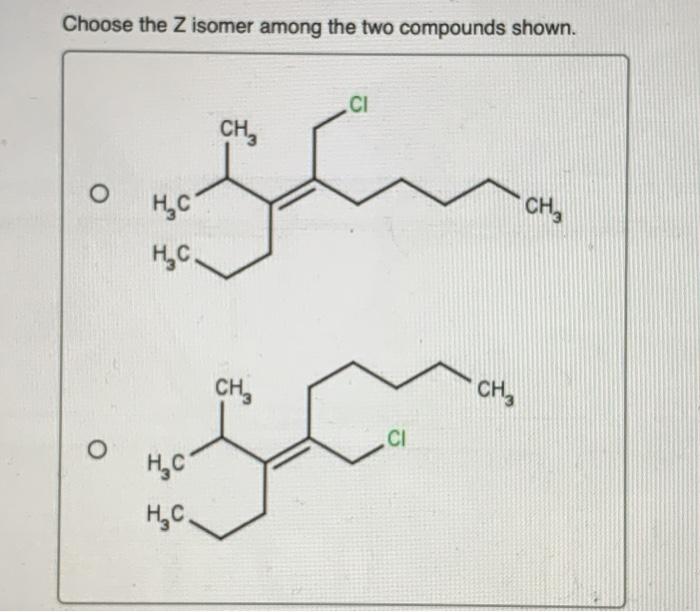
Choose the Z isomer among the two compounds shown sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This guide delves into the intricacies of Z isomers, unraveling their structural characteristics, identification methods, and diverse applications.
Prepare to embark on a journey that illuminates the fascinating world of stereochemistry, where the subtle nuances of molecular arrangements hold profound implications for the properties and behavior of compounds.
Stereochemistry of Z Isomers: Choose The Z Isomer Among The Two Compounds Shown
Stereoisomers are molecules with the same molecular formula but different spatial arrangements of atoms. Cis-trans isomerism is a type of stereoisomerism that occurs when heavy atoms or functional groups are attached to each of the two carbon atoms in a double bond.
Z isomers are cis isomers where the higher priority groups on each carbon atom of the double bond are on the same side of the double bond. This can be determined using the Cahn-Ingold-Prelog (CIP) priority rules.
Identifying Z Isomers, Choose the z isomer among the two compounds shown
The CIP priority rules assign a priority to each atom or group attached to a carbon atom based on its atomic number and the atomic numbers of the atoms directly attached to it. The higher the atomic number, the higher the priority.
To identify Z isomers, follow these steps:
- Identify the two carbon atoms in the double bond.
- For each carbon atom, assign a priority to the atoms or groups attached to it using the CIP rules.
- If the higher priority groups on each carbon atom are on the same side of the double bond, the isomer is Z.
| Priority | Atom/Group |
|---|---|
| 1 | Atom with higher atomic number |
| 2 | Atom with more heavy atom substituents |
| 3 | Atom with more double bonds |
| 4 | Atom with more triple bonds |
Comparison of Z and E Isomers
Z and E isomers have different physical and chemical properties. Z isomers are typically more polar than E isomers because the higher priority groups are closer together. This can affect their solubility, boiling point, and melting point.
The stability of Z and E isomers depends on several factors, including steric hindrance, dipole-dipole interactions, and resonance. In general, Z isomers are more stable when there is less steric hindrance and more dipole-dipole interactions.
Examples of Z and E isomers include:
- Z-2-butene and E-2-butene
- Z-1,2-dichloroethylene and E-1,2-dichloroethylene
- Z-retinal and E-retinal
Applications of Z Isomers
Z isomers are important in biological systems, where they can play a role in enzyme catalysis, protein folding, and cell signaling. In the pharmaceutical industry, Z isomers are often used as drugs because they can have different biological activity than their E isomers.
Z isomers are also used in the agrochemical industry as pesticides and herbicides. In materials science and nanotechnology, Z isomers are being investigated for their potential use in the development of new materials with unique properties.
FAQ Explained
What is the key difference between Z and E isomers?
Z isomers have the higher priority groups on the same side of the double bond, while E isomers have them on opposite sides.
How can I determine the Z/E configuration of a compound?
Use the Cahn-Ingold-Prelog (CIP) priority rules to assign priorities to the groups attached to each carbon atom of the double bond.
What factors influence the stability of Z and E isomers?
Steric hindrance, dipole-dipole interactions, and resonance effects all play a role in determining the relative stability of Z and E isomers.