Delving into the realm of thermodynamics, we explore the heat of vaporization of isopropyl alcohol, a crucial property that governs its transition from liquid to gas. This property plays a pivotal role in its widespread applications, from its use as an antiseptic to its efficacy as a solvent.
Isopropyl alcohol, with its unique molecular structure and intermolecular forces, exhibits a distinct heat of vaporization. Understanding the factors that influence this property allows us to harness its potential effectively.
Heat of Vaporization of Isopropyl Alcohol
Isopropyl alcohol, also known as 2-propanol, is a colorless, flammable liquid with a characteristic pungent odor. It is a common solvent and is used in a variety of industrial and consumer products. The heat of vaporization of isopropyl alcohol is a measure of the energy required to convert one mole of liquid isopropyl alcohol into one mole of gas at its boiling point.
Physical Properties of Isopropyl Alcohol: Heat Of Vaporization Of Isopropyl Alcohol
The molecular formula of isopropyl alcohol is C 3H 8O. It has a molar mass of 60.10 g/mol and a density of 0.786 g/mL at 25 °C. The high heat of vaporization of isopropyl alcohol is due to its relatively strong intermolecular forces.
Intermolecular Forces in Isopropyl Alcohol
The intermolecular forces present in isopropyl alcohol include hydrogen bonding, dipole-dipole interactions, and van der Waals forces. Hydrogen bonding is the strongest of these forces and is responsible for the high boiling point of isopropyl alcohol. Dipole-dipole interactions are also significant, as the isopropyl alcohol molecule has a permanent dipole moment.
Van der Waals forces are the weakest of the intermolecular forces and are responsible for the attraction between nonpolar molecules.
Energy Required for Vaporization
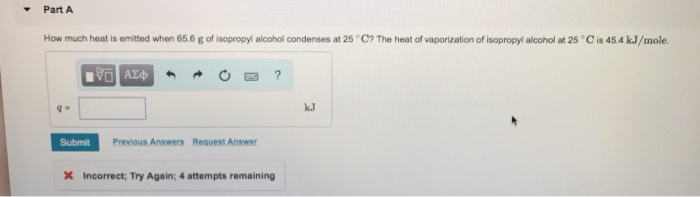
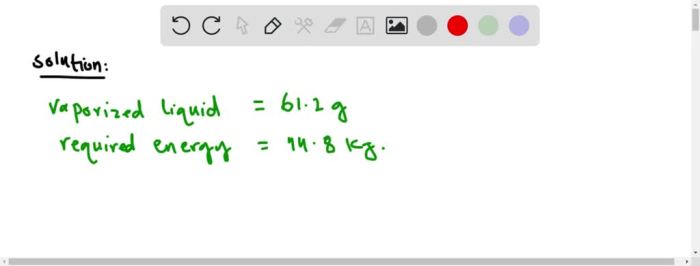
The heat of vaporization of isopropyl alcohol is 44.8 kJ/mol. This means that 44.8 kJ of energy is required to convert one mole of liquid isopropyl alcohol into one mole of gas at its boiling point. The heat of vaporization is a measure of the strength of the intermolecular forces in a liquid.
The higher the heat of vaporization, the stronger the intermolecular forces.
Applications of Heat of Vaporization
The high heat of vaporization of isopropyl alcohol makes it useful in a variety of applications, including:
- Antiseptic and disinfectant: Isopropyl alcohol is used as an antiseptic and disinfectant because it kills bacteria and viruses. The high heat of vaporization of isopropyl alcohol helps to kill microorganisms by denaturing their proteins.
- Solvent: Isopropyl alcohol is a good solvent for a variety of organic compounds. It is used in the manufacture of paints, varnishes, and other products.
- Deicing agent: Isopropyl alcohol is used as a deicing agent for aircraft and windshields. The high heat of vaporization of isopropyl alcohol helps to melt ice and snow.
Comparison to Other Alcohols, Heat of vaporization of isopropyl alcohol
The heat of vaporization of isopropyl alcohol is higher than that of methanol and ethanol. This is because isopropyl alcohol has a higher molecular weight and a more complex molecular structure. The table below shows the heat of vaporization values for different alcohols:
| Alcohol | Heat of Vaporization (kJ/mol) |
|---|---|
| Methanol | 35.3 |
| Ethanol | 38.6 |
| Isopropyl alcohol | 44.8 |
Common Queries
What is the significance of heat of vaporization?
Heat of vaporization quantifies the energy required to convert a liquid into a gas at a constant temperature. It provides insights into the strength of intermolecular forces within the liquid.
How does isopropyl alcohol’s molecular structure affect its heat of vaporization?
Isopropyl alcohol’s branched molecular structure results in weaker intermolecular forces compared to linear alcohols. This contributes to its lower heat of vaporization.
What practical applications utilize the high heat of vaporization of isopropyl alcohol?
Its high heat of vaporization makes isopropyl alcohol an effective antiseptic and disinfectant, as it evaporates quickly, carrying away microorganisms.